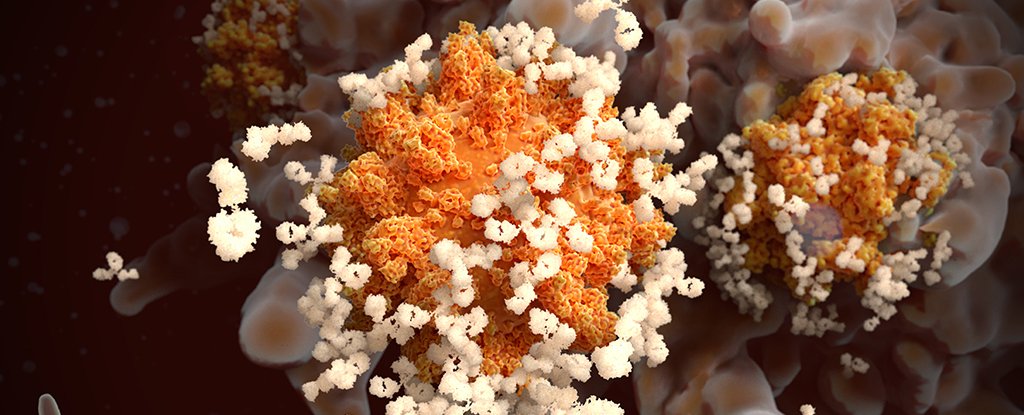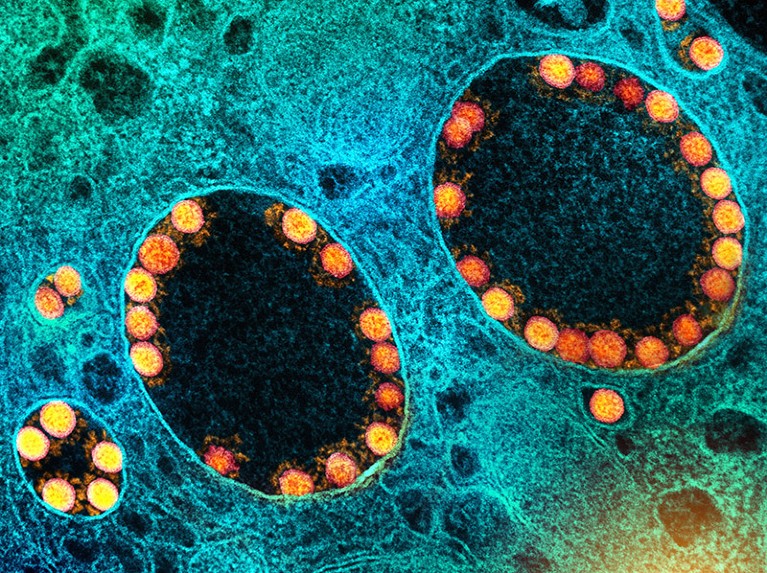
Particles of SARS-CoV-2 (yellow; artificially coloured) infect cells in the lining of the nasal passage.Credit: National Institute of Allergy and Infectious Diseases/National Institutes of Health/SPL
Delivery of COVID-19 vaccines directly to the lungs and nose can stop SARS-CoV-2 infections in their tracks, according to a trio of new studies in monkeys. The research offers a boost to the wave of ‘mucosal’ COVID-19 vaccines now in development — and provides clues about how they might be improved.
Until now, there has been little evidence that mucosal vaccines, which are taken by nose or mouth, shield people against infection any better than existing COVID-19 jabs do. Even so, some countries have already approved such vaccines, and key trials are under way in the United States, with others set to start.
Together, the studies show that how and where vaccines are delivered can have a profound effect on the immunity generated and the protection conferred. The latest results also raise hopes that mucosal vaccines that offer ‘sterilizing’ immunity — complete blockage of infection — could become a reality.
“These studies are showing you can get near sterilizing immunity,” says Akiko Iwasaki, an immunologist at the Yale School of Medicine in New Haven, Connecticut. “It’s not complete science fiction to think about developing vaccines that would stop transmission and infection.”
Jabs fall short
COVID-19 jabs saved millions of lives during the pandemic and continue to do a good job of keeping at-risk individuals out of hospital. But even updated versions of existing injected vaccines offer scant and short-lived protection against infection, scientists say.
Researchers think this is because injected intramuscular vaccines do a good job of stopping SARS-CoV-2’s spread deep in the lungs, but are much less effective in the rest of the airway, where infections probably begin. Mucosal COVID-19 vaccines aim to trigger the production of antibodies and other immune players in the mucous membranes, or mucosa, that line the nose and the rest of the respiratory tract, in the hope that this will boost protection there.
Dozens of mucosal COVID-19 vaccines are in development (see ‘High hopes’) and several have been approved in countries including China and India. But according to an 8 December report by the London-based data and analytics firm Airfinity, the efficacy of existing mucosal COVID-19 vaccines has been disappointing and the available data suggest that “they do not offer a meaningful increase in protection against infection”.

Source: Airfinity
However, the latest studies in monkeys and other laboratory animals offer hints on how these vaccines might be improved. A team led by Dan Barouch, a vaccine scientist at Beth Israel Deaconess Medical Center in Boston, Massachusetts, tried two approaches in monkeys that had previously received COVID-19 jabs: squirting a liquid vaccine into the animals’ noses, or applying it directly to their tracheae1.
Only the trachea-delivered vaccine substantially boosted mucosal immunity and protection against SARS-CoV-2 infection. “We think that the problem with intranasal delivery is that most of the vaccine is either swallowed or sneezed out,” Barouch says. The results were published in Nature on 14 December.
Biochemical engineer Wei Wei at the University of Chinese Academy of Sciences in Beijing and his colleagues created a nanoparticle vaccine that includes a fragment of a SARS-CoV-2 protein, and delivered it as an aerosol into the lungs of mice, hamsters and monkeys. This triggered a strong mucosal immune response — and stymied the spread of SARS-CoV-2 in vaccinated hamsters housed in cages next to one another, the researchers reported in Nature2 on 13 December. Both this study and that by Barouch’s team hint that vaccines applied more deeply to the respiratory system outperform nasal sprays.
China’s COVID vaccines have been crucial — now immunity is waning
In a third study, a team led by Robert Seder, a vaccinologist at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland, reached a slightly different conclusion. His group used devices approved to deliver other medicines to give monkeys a booster dose of mucosal COVID-19 vaccine either by way of the nose or as an inhaled aerosol delivered through the mouth and nose. Then they exposed the animals to SARS-CoV-2.
Monkeys that had received a booster dose with either device developed much lower levels of the virus in their upper airways than did monkeys that had received a booster jab, even though the animals were exposed to the virus five months after the booster. Such durability, Seder says, is “striking”. The results were posted on the preprint server bioRxiv on 8 November and have not yet been peer reviewed.
Straight to the lungs
Delivering aerosolized vaccines to the lungs might be the best strategy for mucosal COVID-19 vaccines, says Zhou Xing, a vaccine immunologist at McMaster University in Hamilton, Canada. But he adds that simple, hand-held delivery devices might need to be developed for such vaccines to be widely deployed.
There is also a need to optimize the vaccines themselves, says Seder. Many of the current injectable vaccines contain SARS-CoV-2 RNA, but Seder’s team has found that the RNA formulations used in those vaccines aren’t effective when inhaled.
The vaccines that Seder’s and Barouch’s teams tested, as well as the mucosal vaccines approved in China and India, are based instead on bioengineered adenoviruses that carry snippets of genetic material from SARS-CoV-2. The US government is supporting trials of mucosal vaccines based on other viruses: one that harnesses Newcastle disease virus, which infects birds, and a second based on a strain of SARS-CoV-2 that has been altered to replicate too slowly to cause disease.
China and India approve nasal COVID vaccines — are they a game changer?
The protein-based vaccine that Wei’s team developed has the advantage that it can be stored at room temperature in a powdered form, reducing storage and transportation costs, Wei says.
Showing that mucosal vaccines actually work — and work better than existing jabs do — is another challenge facing the field. One possibility for testing these products is a human challenge trial, in which participants are intentionally infected with a virus. Seder expects it to take at least another two or three years to develop successful mucosal vaccines for COVID-19.
But the burst of interest in these vaccines has wider relevance, say scientists. If we can learn how to trigger mucosal immunity against SARS-CoV-2, it should be possible to do the same for influenza, respiratory syncytial virus and infections we might not yet know about. “It’s a worthy goal,” says Hyon-Xhi Tan, an immunologist at the University of Melbourne, Australia, “especially for respiratory viruses that may pop up in the future with pandemic potential.”

Powered by Jennyfar.com